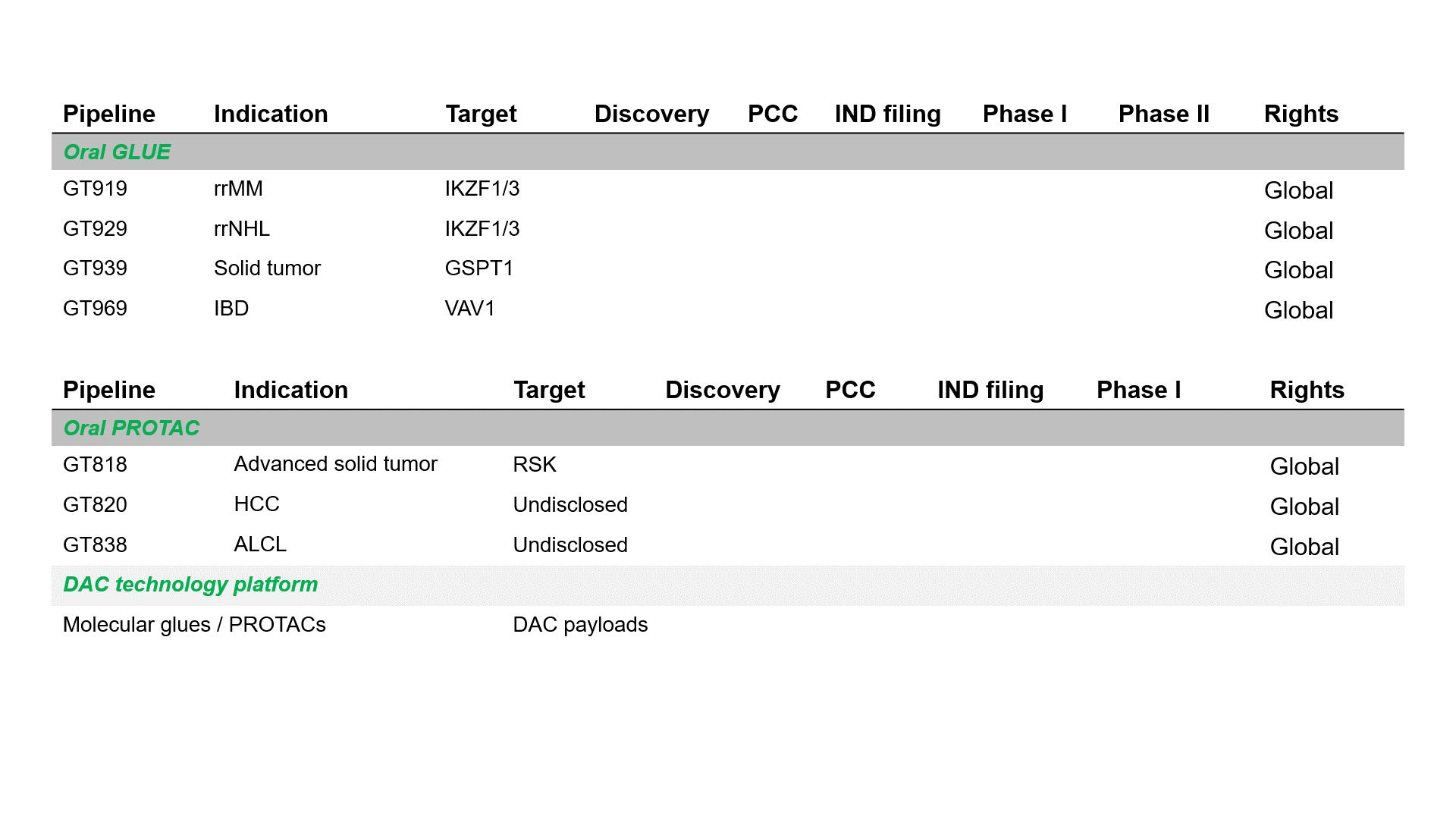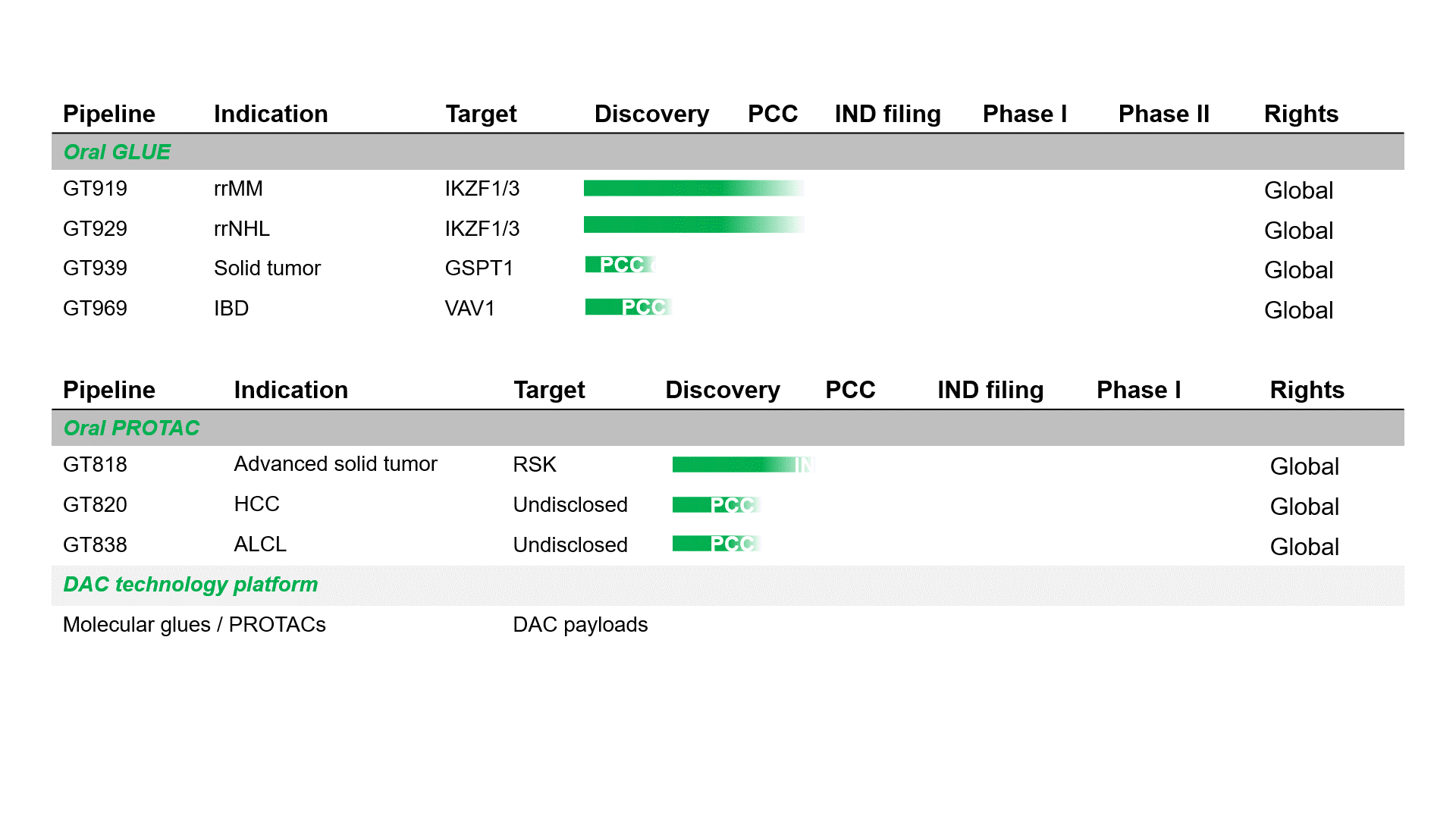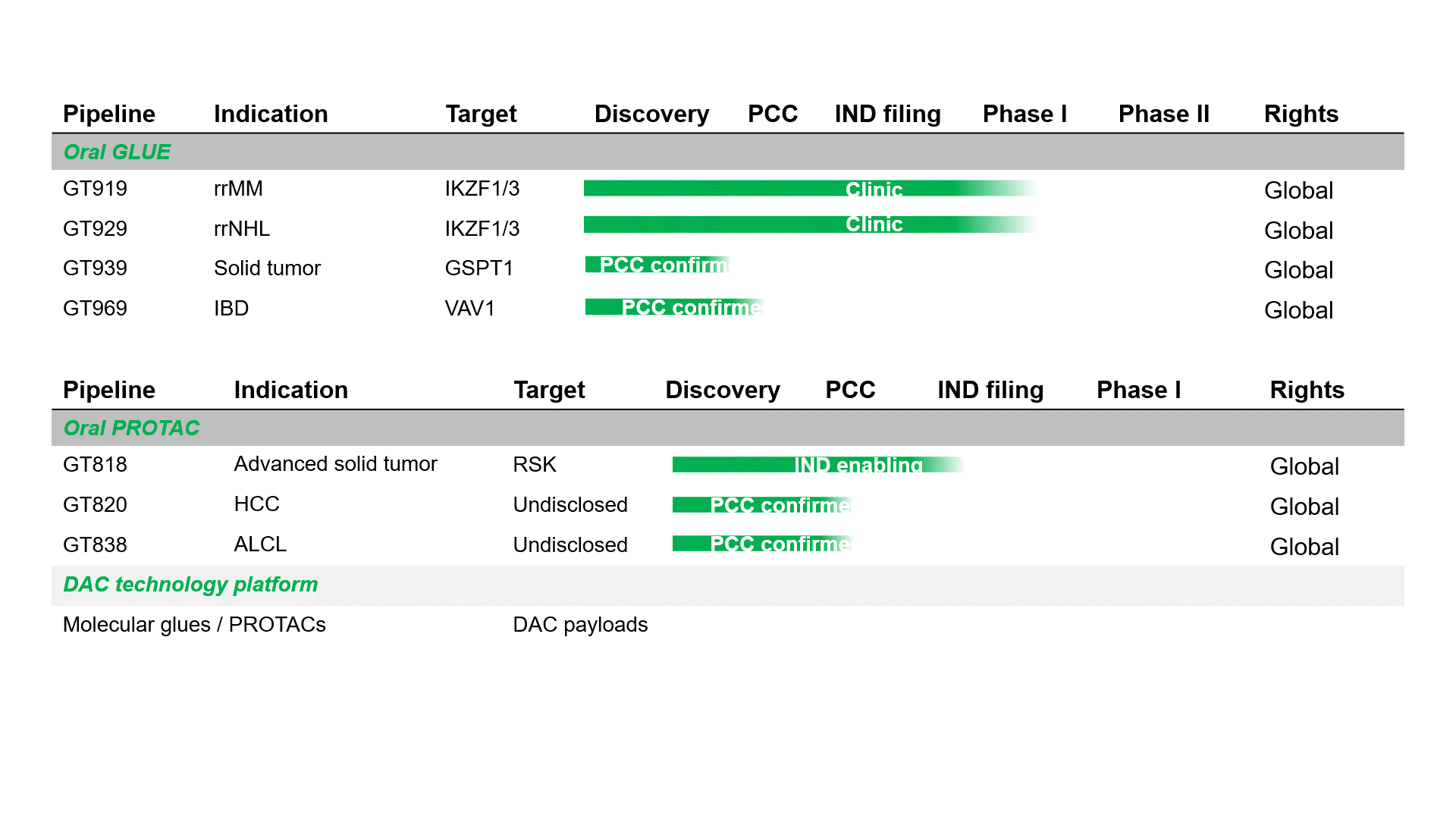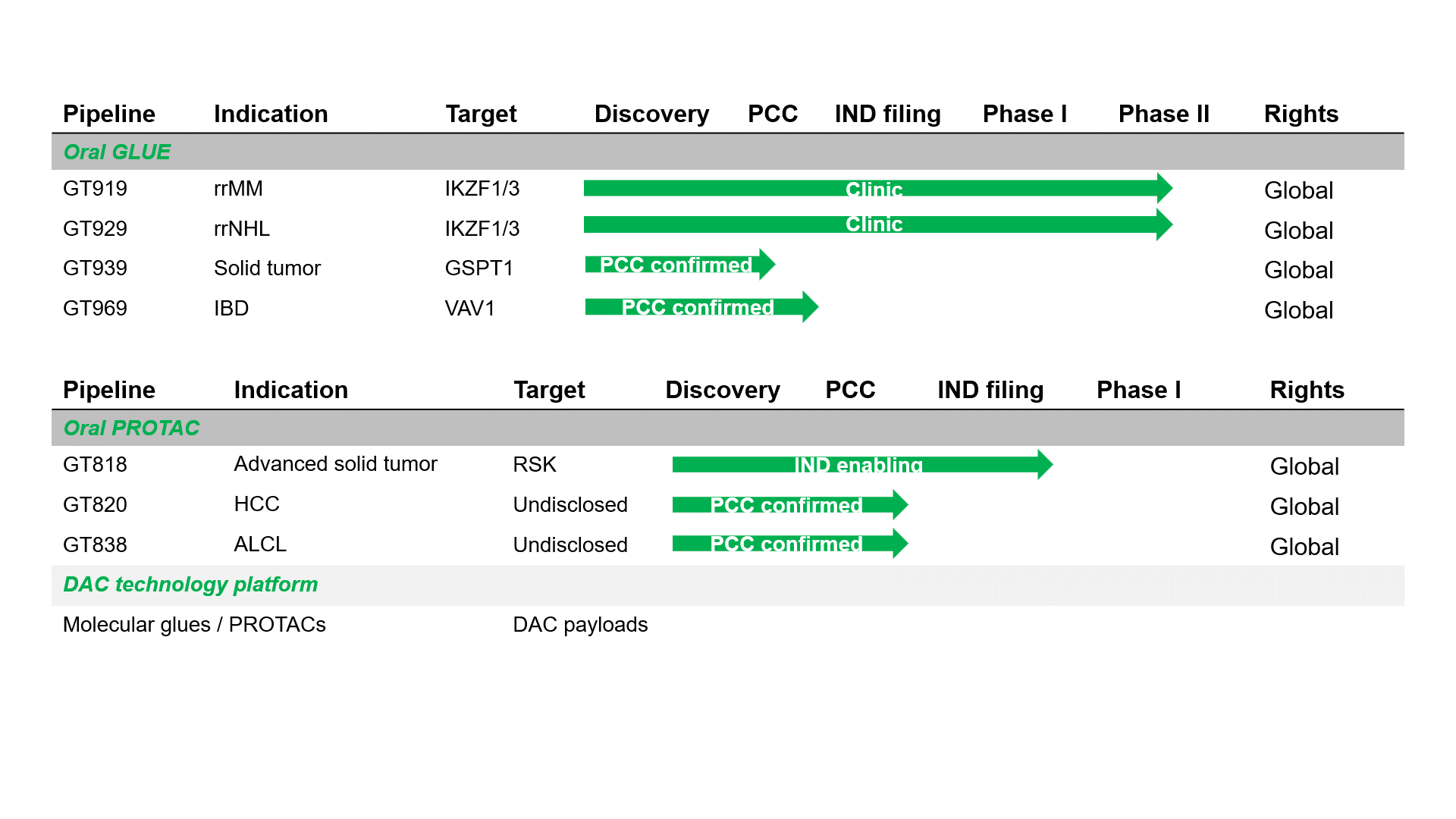
Pipeline
GT919
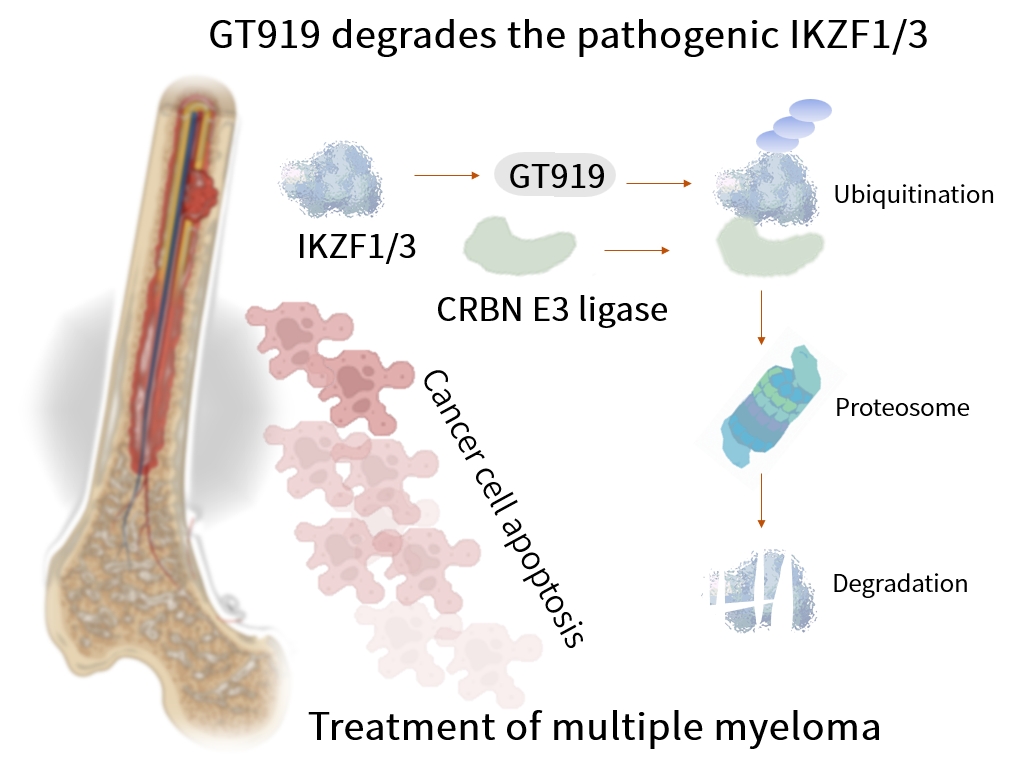
GT919 is a disruptive IKZF3 degrader developed by Gluetacs Therapeutics with proprietary IP. Designed to overcome the limitations of existing agents, GT919 achieves greater selectivity and potency in target degradation while minimizing off-target toxicities. Comprehensive druggablilty studies highlight GT919 as an innovative and differentiated program, developed with a strategy distinct from “fast-follow” or “me-too” approaches. Key features include enhanced enrichment in bone marrow lesions and the ability to penetrate the blood–brain barrier, properties that broaden its therapeutic potential.Both preclinical and clinical data demonstrate superior safety and therapeutic benefit compared with CC-92480. Notably, hematologic toxicities observed in clinical settings have been only transient, highlighting GT919’s favorable safety profile.
GT929
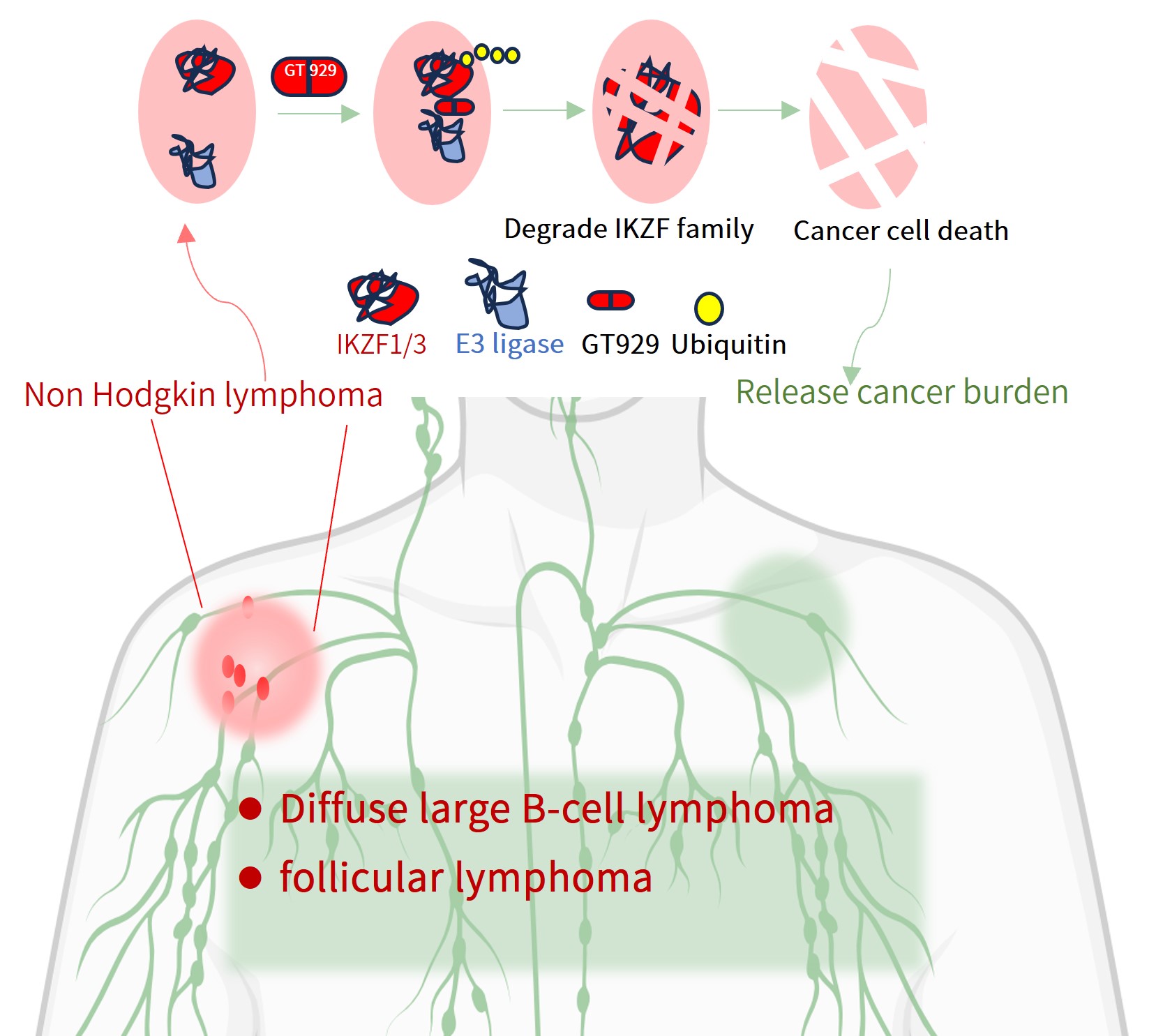
GT929 is a next-generation IKZF3/1 degrader developed by Gluetacs Therapeutics with proprietary IP. GT929 is highly selective and potent in targeted degradation while reducing off-target toxicities.Preclinical studies show GT929 as an innovative and differentiated program, developed with a strategy distinct from “fast-follow” or “me-too” approaches. Its unique profile includes enhanced enrichment in lymphatic lesions and the ability to cross the blood–brain barrier, properties that expand its therapeutic reach.In clinical studies, GT929 has demonstrated superior safety and efficacy relative to CC-99282, reinforcing its potential as a best-in-class therapy for hematologic malignancies.
GT818
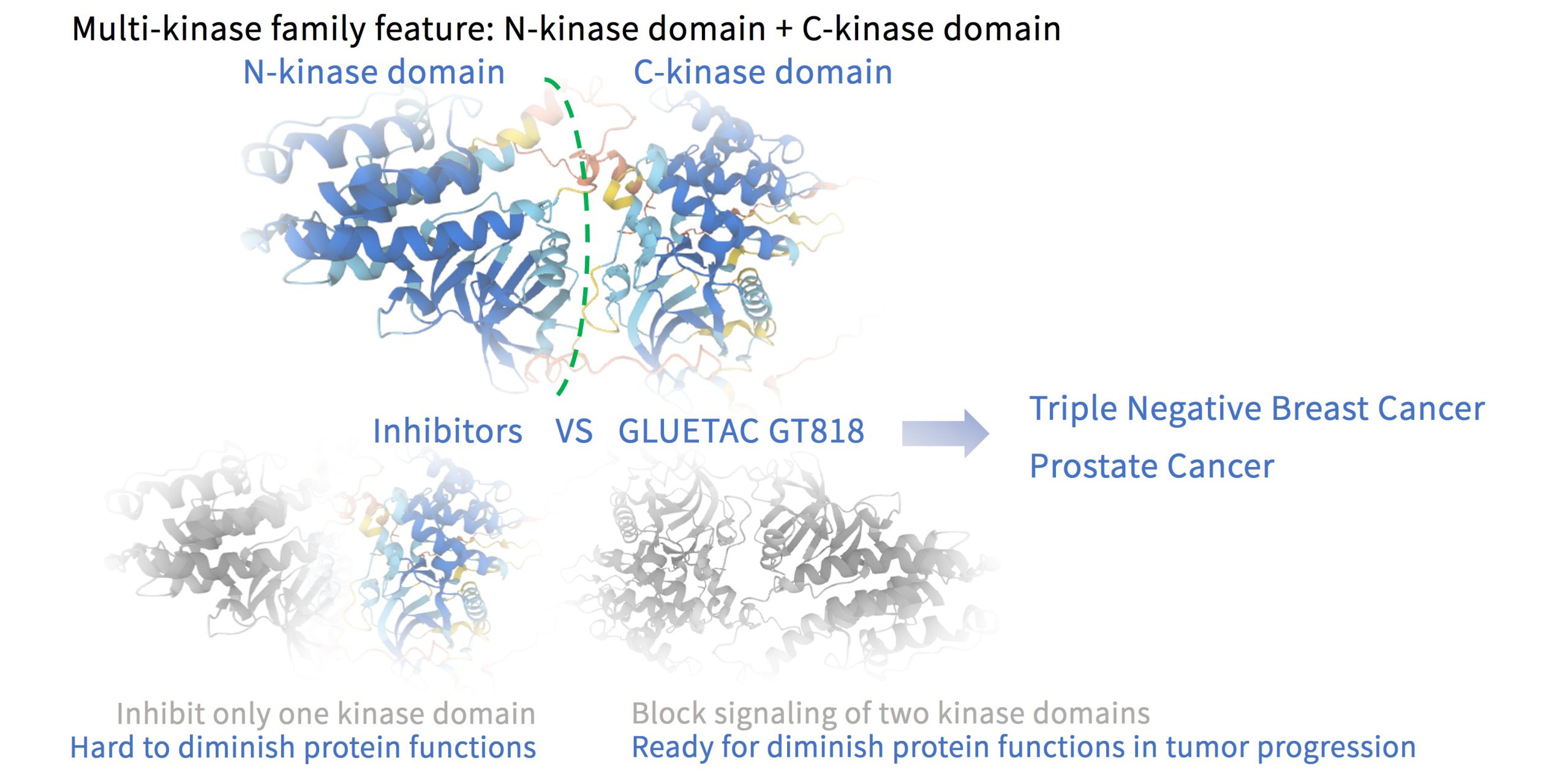
GT818 is a first-in-class oral RSK degrader developed by Gluetacs Therapeutics with proprietary IP. In preclinical models, GT818 demonstrates a high selectivity for RSK, wide safety margin, significant enrichment in solid tumor tissues, and a degree of blood–brain barrier penetration.We are developing GT818 across multiple solid tumor indications including breast, prostate, non-small cell lung, pancreatic, and colorectal cancers with the goal of delivering differentiated options in solid oncology.
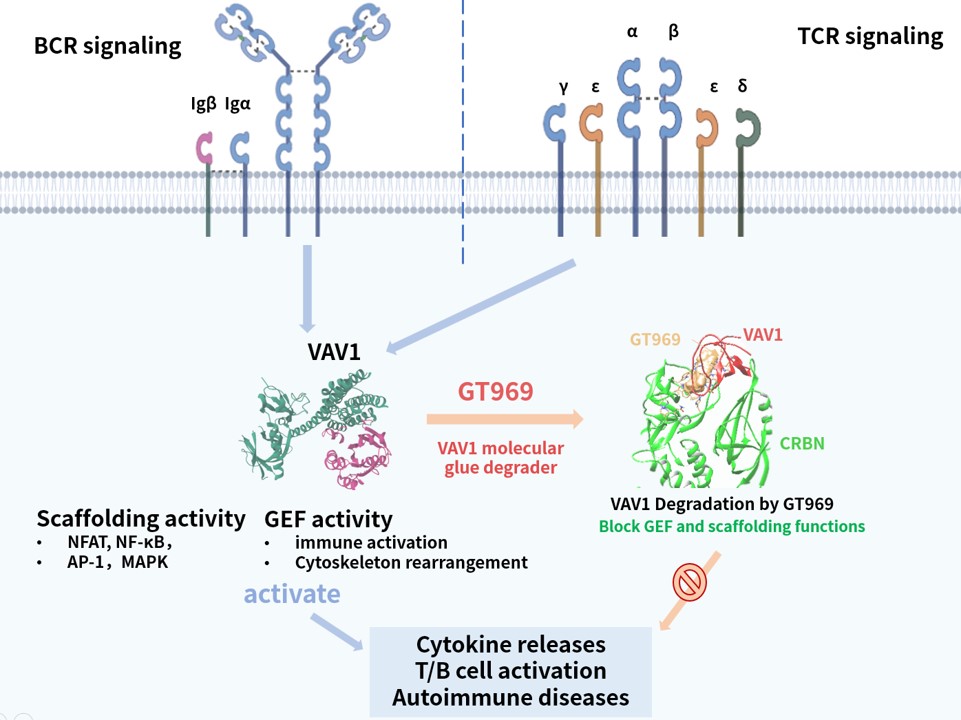
GT969
GT969 is a unique, first-in-class oral VAV1 molecular glue degrader developed by Gluetacs Therapeutics with proprietary IP. By selectively degrading a previously undruggable scaffold protein VAV1, GT969 modulates the immune system and introduces a novel approach to autoimmune disease treatment.Preclinical research shows that GT969 has enhanced enrichment in disease-relevant tissues such as the gut, skin, and lungs, differentiating it from MRT6160, a clinical-stage competitor. In autoimmune disease models including inflammatory bowel disease, psoriasis, and arthritis, GT969 demonstrates efficacy that outperforms MRT6160 and other regulatory-approved therapies.With its novel mechanism, oral delivery, and targeted tissue enrichment properties, GT969 represents a differentiated opportunity to reshape the treatment landscape for autoimmune diseases and has the potential to become a transformative therapy in this space.