Gluetacs Therapeutics and International Team Reveal a New Mechanism by Which FER Kinase Regulates Head and Neck Squamous Cell Carcinoma Invasion, Providing a Novel Strategy to Overcome Resistance to Targeted Therapy
ON:2025-10-22 TAGS:GLUETACS THERAPEUTICS
Head and neck squamous cell carcinoma (HNSCC) accounts for 90% of all head and neck cancers. HPV-negative HNSCC exhibits extensive local invasion of surrounding tissues and patients often experience loco-regional relapse and local/distance metastasis. Current treatment strategies for locally advanced or non-resectable tumors targeting single growth factor receptors offer limited therapeutic benefit. There is an urgent need to identify a target that can simultaneously inhibit multiple cancer-related growth factor receptors, and to develop new therapeutic strategies to improve HNSCC treatment outcomes.
As a co-corresponding institution, Gluetacs Therapeutics, together with the University Medical Center Utrecht and other international collaborators, has published a research article in the prestigious journal Neoplasia titled “FER Kinase Governs Invasive Growth of Head and Neck Squamous Cell Carcinoma through Dynamic Control of Growth Factor Receptor Activity.” This study is the first to identify FER, a non-receptor tyrosine kinase, as a critical driver of invasive growth in HNSCC. By dynamically modulating the activity of multiple growth factor receptors, including EGFR and MET, FER drives tumor invasion and metastasis in HNSCC, highlighting its potential as a promising therapeutic target.
Clinical studies have shown that FER is highly expressed in HNSCC tissues and is significantly associated with lymph node involvement and a candidate prognostic marker for poor prognosis in patients. Using patient-derived tumor organoid models, researchers confirmed that FER is essential for the activation of EGFR and MET and the transmission of their downstream MAPK signaling pathways. Mechanistic investigations further showed that FER not only regulates the initial phosphorylation and activation of growth factor receptors at the cell membrane, but also sustains signaling within endosomal vesicles by modulating the endocytic trafficking of ligand–receptor complexes. Through this dual regulation of receptor activity, FER serves as a critical molecular node for maintaining oncogene addiction and mediating resistance to targeted therapy in HNSCC.
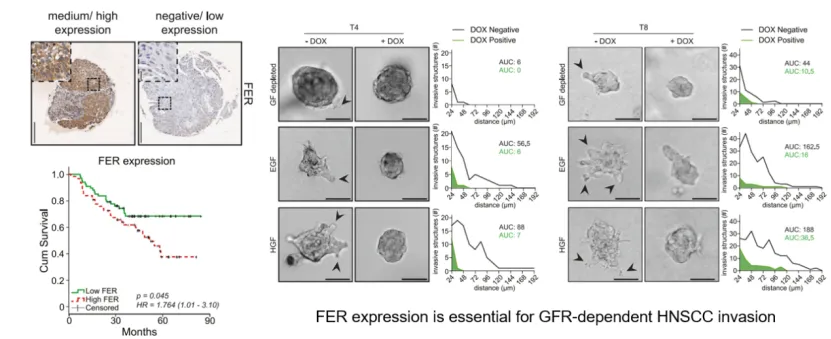
Fig. 1. FER is highly expressed in HNSCC and affects prognosis; knockdown of FER suppresses tumor cell invasion in an organoid model.
To verify the therapeutic potential of FER as a drug target, the research team designed and synthesized a FER-specific PROTAC degrader for target validation and pharmacodynamic studies. In both in vitro organoid and in vivo xenograft models, the FER-PROTAC efficiently degraded the target protein, markedly inhibited growth factor–induced tumor cell invasion, and effectively suppressed tumor growth in vivo.
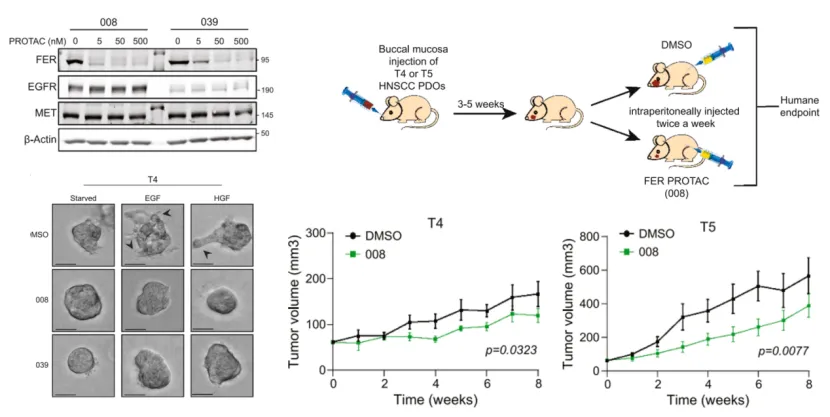
Fig. 2. Both compounds 008 and 039 significantly degrade FER; they suppress tumor invasion in an organoid model; compound 008 demonstrates potent anti-tumor activity in PDX models T4 and T5.
This approach overcomes the limited efficacy of single-target therapies against EGFR and MET, providing a novel concept and preclinical foundation for translational research in HNSCC targeted therapy, while also offering valuable insights for treating other highly invasive cancers.
This study not only elucidates the central role and novel mechanism of FER kinase in driving the invasive growth of HNSCC, but also demonstrates that targeted degradation of FER using PROTAC technology holds great promise for the treatment of HNSCC. As the co-corresponding institution, Gluetacs Therapeutics, together with its academic collaborators, has published another impactful study, further highlighting the technological depth and translational potential of the GlueTacs® platform, showcasing its academic significance and commercial potential.
https://authors.elsevier.com/sd/article/S1476-5586(25)00121-6